Measuring the specific latent heat of fusion of Ice YouTube

Solved Latent Heat of Fusion Lab Instructions In this lab
The phase diagram for carbon dioxide shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the carbon dioxide boiling point with changes in pressure.
Latent Heat Of Water In Calories / 14 4 Phase Change And Latent Heat The latent heat for
The latent heat of fusion refers to the phase change between states of solid and liquid. Here, heat actually refers to the transfer of heat energy between the objects. Thus, the latent heat of fusion encompasses the process of adding heat to melt some solid. The formula for Latent heat of fusion:

Specific Latent Heat Of Evaporation Formula Specific Heat Capacity And Latent Heat Enthalpy
Latent heat of evaporation (at 100°C): 40.657 kJ/mol = 2256 kJ/kg = 970 Btu (IT)/lb C): 999.975 kg/m = 1.9403 slug/ft = 8.34519 lb /gal (US) Melting temperature (at 101.325 kPa): 0 °C = 32°F pH (at 25°C): 6.9976

Latent heat YouTube
Step 3: Calculate the latent heat of fusion. Calculate the specific latent heat of fusion of ice to water using the equation ΔE = LΔm. ΔE = Energy supplied by the heater = 10, 000 J; L = Specific latent heat of fusion; Δm = mass of ice; Rearrange the equation; So, = 340136 J kg-1 = 340 000 J
Phase Change Requires Heat FD2021 Fundamentals of Fire and Combustion on Guides
18.7: Latent Heat of Fusion. The most straightforward method for measuring the specific latent heat L of ice is to drop a lump of. Ice of mass m and specific latent heat L at its melting point T0 into a. Calorimeter of mass MC and specific heat capacity CC and initial (warm) temperature T2, a mass MW of Water of specific heat capacity CW at the.
Latent Heat of Fusion Definition, Formula, Examples & FAQs
Latent heat is measured in units of J/kg. Both L f and L v depend on the substance, particularly on the strength of its molecular forces as noted earlier. L f and L v are collectively called latent heat coefficients.They are latent, or hidden, because in phase changes, energy enters or leaves a system without causing a temperature change in the system; so, in effect, the energy is hidden.

unit08 latent heat of fusion YouTube
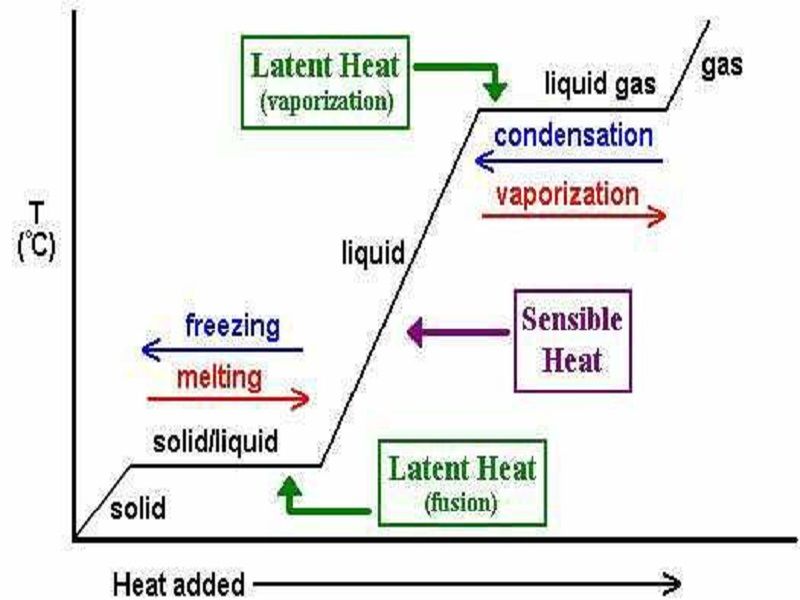
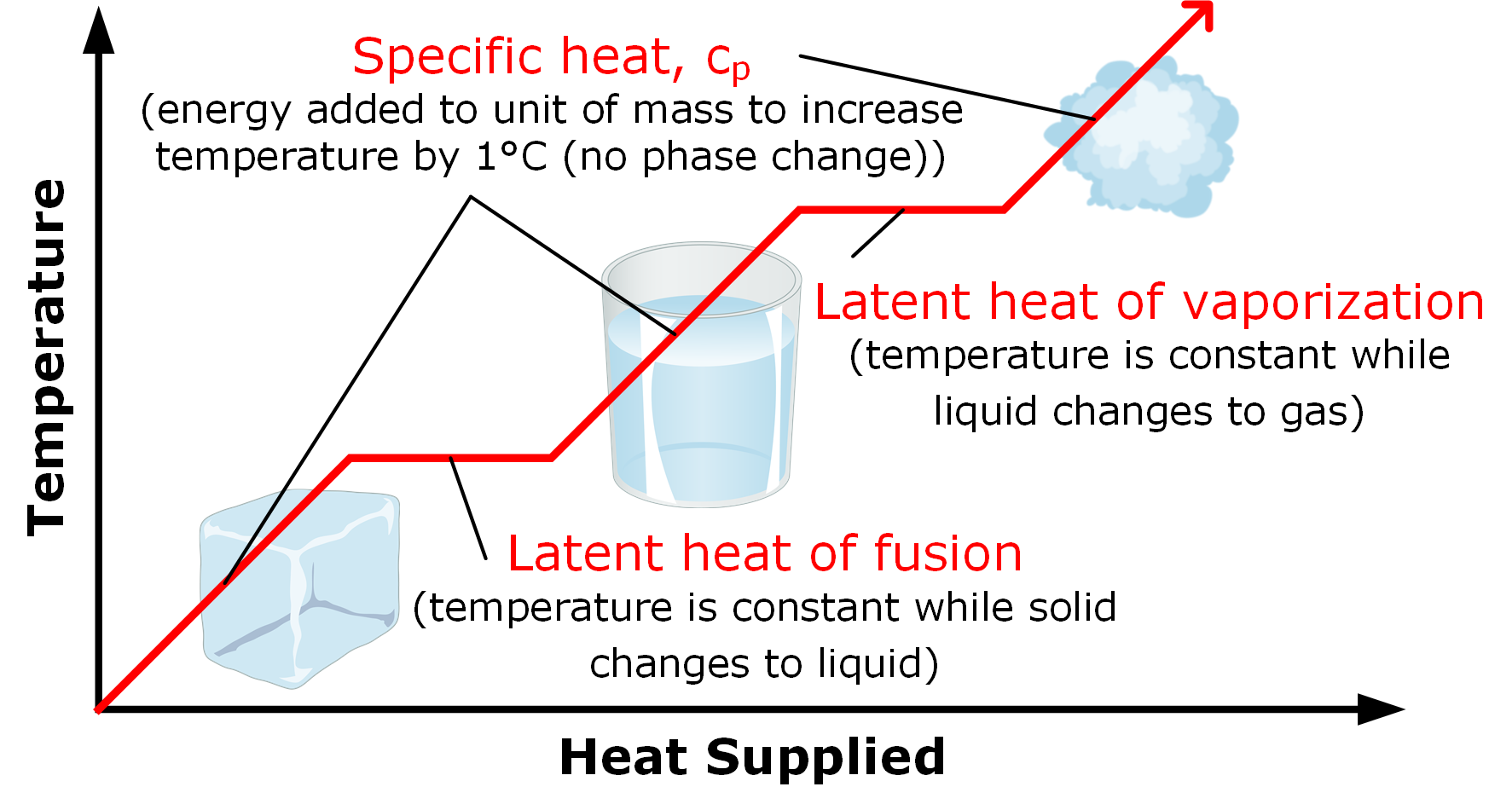
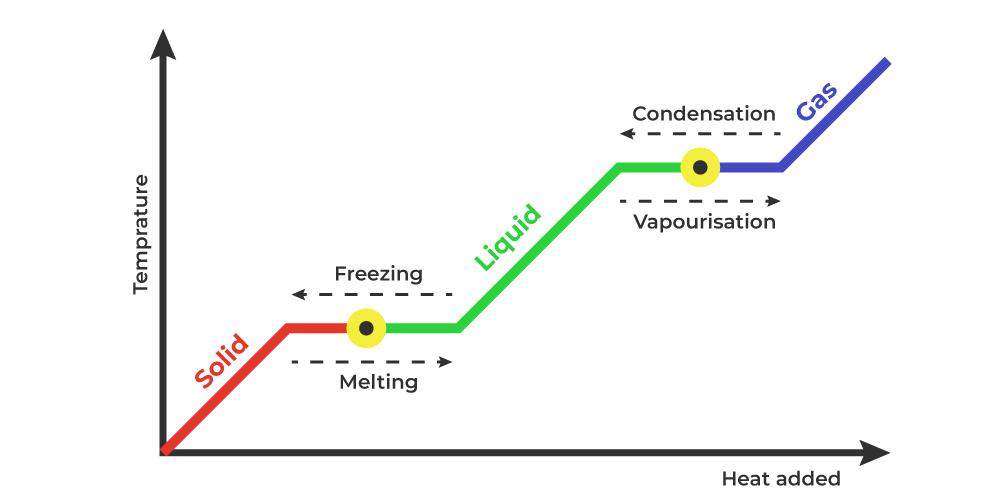
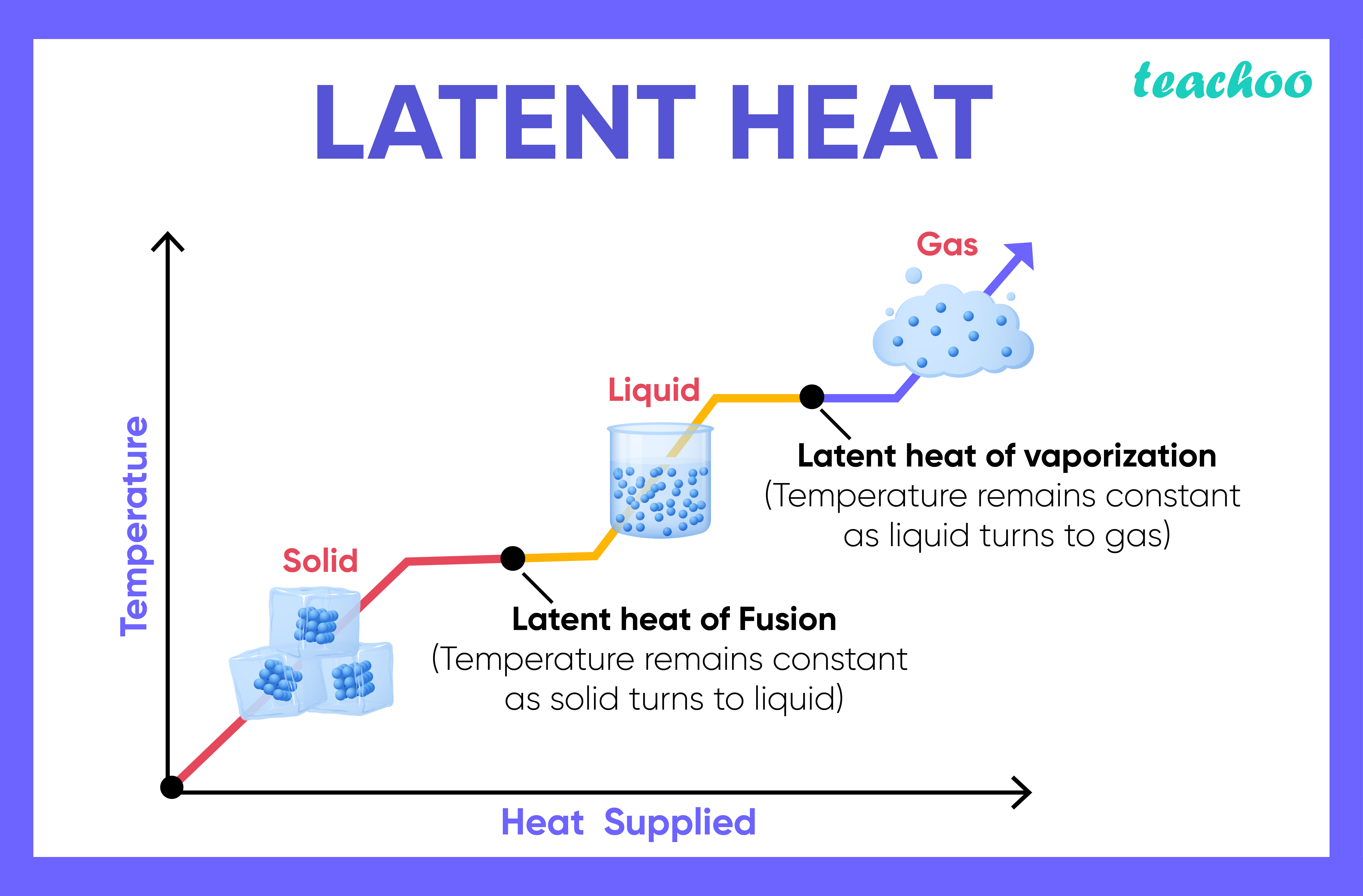
Phase changes Transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the specific heat.If heat were added at a constant rate to a mass of ice to take it through its phase changes to liquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization) would.

PPT Change of State PowerPoint Presentation, free download ID6691426
Because this energy enters or leaves a system during a phase change without causing a temperature change in the system, it is known as latent heat (latent means hidden ). The three phases of matter that you frequently encounter are solid, liquid and gas (see Figure 11.8 ).

Four Differencebetween latent heat of fusion and latent heat of vaporization Brainly.in
In Chemistry, Latent heat is heat that occurs when a substance changes physically without changing its temperature as a result of the release or absorption of energy. Ice, Water have different latent heat of fusion. Not only this but it is also having a specific latent heat of fusion.

Latent Heat Of Vaporization Electricity detailed contents / When a material in liquid state
What the heck is dry ice and why is it so spooky? Learn this and more when we investigate phase changes and phase diagrams!Watch the whole General Chemistry.

draw labelled diagram of the experimental set up to the latent heat of fusion of ice Brainly.in
The latent heat of fusion of the eutectic, Δ fus H E, is estimated from the total change in entropy on fusion, Δ fus S E. To evaluate the thermodynamic models, we compared the values estimated by these models with experimental values reported in the literature, and values obtained here for two eutectic mixtures of fatty acids: a eutectic.
For any substance, why does the temperature remain constant during the
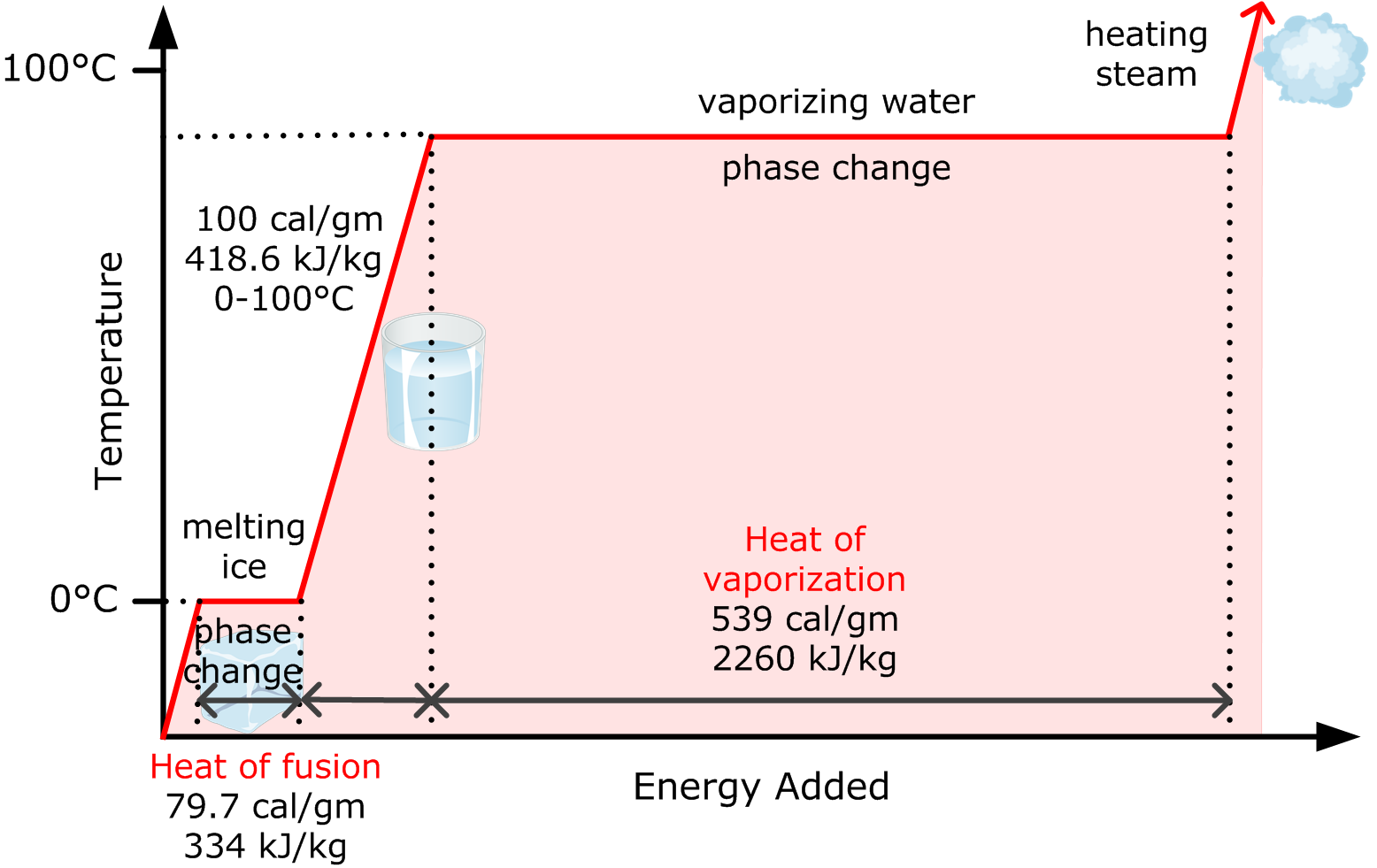
Using the equation for a change in temperature and the value for water from Table 14.2, we find that Q = mLf =(1.0kg)(334kJ/kg)= 334 kJ Q = mL f = ( 1. 0 kg) ( 334 kJ/kg) = 334 kJ is the energy to melt a kilogram of ice. This is a lot of energy as it represents the same amount of energy needed to raise the temperature of 1 kg of liquid water.

Latent heat of fusion for common wrought alloy families. Download Scientific Diagram
Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically in the form of heat) in order to trigger a change in its physical state and convert it into a liquid (when the pressure of the environment is kept constant).

Latent heat of fusion for common wrought alloy families. Download Scientific Diagram
construct a phase diagram by specifying the phase(s) observed at particular P,T combinations: t. the latent heat involves a change in internal energy, plus work in expanding the volume. Examples of latent heats: material fusion, L f (J / kg) vaporization, L v (J / kg) water 33.5 x 104 22.6 x 105 ethyl alcohol 10.8 x 104 8.55 x 105
Latent Heat Water My XXX Hot Girl
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation.. Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or.

draw labelled diagram of the experimental set up to the latent heat of fusion of ice Brainly.in
I explain the definition of Latent Heat and how it's used in the equation Q = mL, how to identify the latent heat of fusion, vaporization, and sublimation, a.